It is safe to say that COVID-19 has been the number one concern in healthcare in the United States during 2020. Since January, John Hopkins University Center for Systems Science and Engineering reported that health authorities have identified more than 8 million Covid-19 cases and 220,046 deaths throughout the United States. With cases on the rise, this can bring up many questions like:
- What does Pharmacologic Management look like?
- What are the Medication Costs to treat COVID-19?
- How has it affected Medical Care?
- How will this affect the future?
That is why today we are looking at each of these questions and giving you clear insight into the data surrounding the coronavirus.
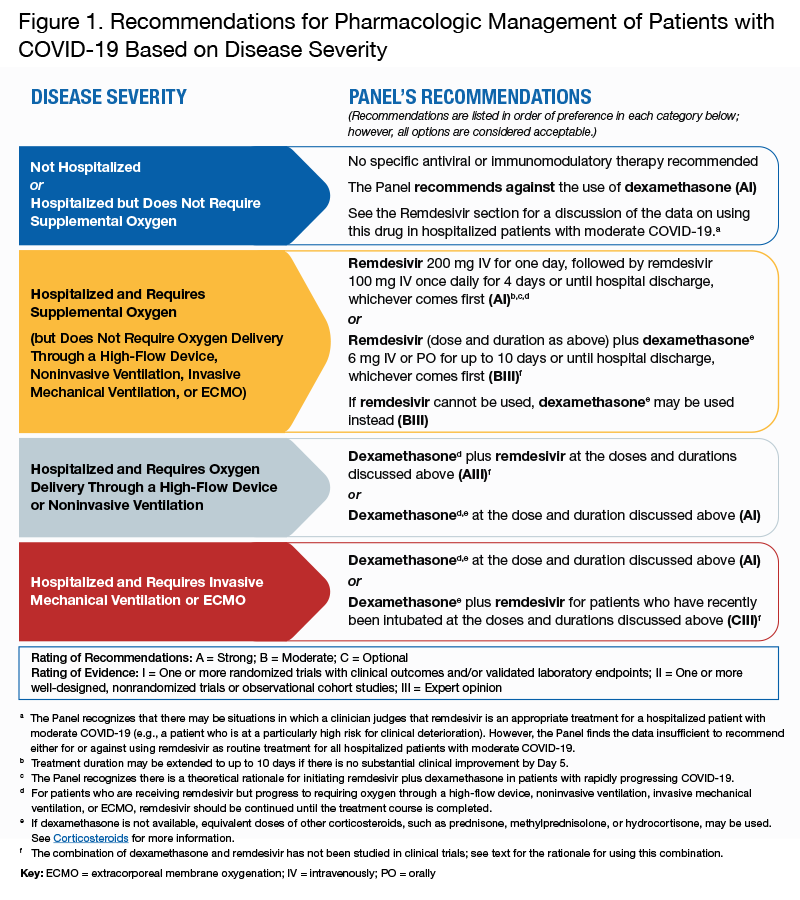
The Pharmacologic Management of patients with COVID-19 uses a number of investigational agents and drugs that are being studied in clinical trials for the treatment of the virus and associated complications. In the figure below provided by the National Institutes of Health, share recommendations for pharmacologic management of patients with COVID-19 based on severity.
Medication costs for COVID-19 patients hospitalized in the United States have dropped sharply since May, reflecting advances in treatment, shorter stays, and use of cheaper generic drugs. But costs may rise again as hospitals start to pay for Gilead Sciences Inc’s remdesivir. Research by the health data firm IllumiCare and exclusively shared with Reuters found that hospitals spent $1,090 per COVID-19 patient on medication in July. That was down from $3,011 in May among more than 50 hospitals in 10 states that were analyzed. Several factors drove down the number. The average length of stay for COVID-19 patients declined by nearly 30%, from 9.6 days in April to 6.8 days in July, the hospital data show. And the number of medications used dropped by 22%, from nearly 20 individual drugs in April to 15.4 drugs in July. Remdesivir, which helped speed up patients’ recovery in a U.S. trial, won emergency-use authorization in May from U.S. regulators.
Pharmacy costs could increase significantly in the months ahead because of remdesivir. Gilead donated early doses but has begun charging. It has said the price for commercially insured patients is $3,120 per treatment course and $2,340 for patients on Medicare. In addition to remdesivir, hospital costs also may rise because of the increased use of tocilizumab, an anti-inflammatory drug widely used to treat arthritis. Hospital use jumped 29% among COVID-19 patients during July compared with the month earlier. Tocilizumab costs more than $2,200 per patient and is one of a class of drugs that includes Roche’s Actemra. The most frequently prescribed drug for COVID-19 patients was the anticoagulant enoxaparin. It was given to 50% of inpatients last month at a cost of $322 per patient, the data show.
The pandemic has impacted medical care received by adults. It is estimated that 41 percent delayed or avoided medical care during COVID-19. About 12 percent reported avoiding urgent or emergency care and 31.5 percent reported avoiding routine care because of concerns about COVID-19. More than half of adults aged 18 to 24 (57.2 percent) and unpaid caregivers for adults (64.3 percent) said they avoided care. Close to 55 percent of people with two or more underlying medical conditions said they avoided care.
So, how will this influence the years ahead?
A drug pricing forecast predicts a 3.29% increase for pharmaceutical purchases by health systems in 2021, driven by ongoing disruptions due to the coronavirus disease 2019 (COVID-19) pandemic and enduring market trends, according to a press release. The report said that the COVID-19 pandemic has created many uncertainties, including variations in medication use trends and the impact of total expenditures, which could have a significant effect in the coming year. The report found that the top 10 COVID-19-related medications saw $200 million in increased spending during March and April 2020. Hydroxychloroquine specifically saw an 1132% increase during that period.
Vaccines typically require years of research and testing before reaching the clinic, but scientists are racing to produce a safe and effective coronavirus vaccine by next year. Researchers are testing 46 vaccines in clinical trials on humans, and at least 91 preclinical vaccines are under active investigation in animals. Work began in January with the deciphering of the SARS-CoV-2 genome. The first vaccine safety trials in humans started in March, and now 10 have reached the final stages of testing. Some of these trials will fail, and others may end without a clear result. But a few vaccines may succeed in stimulating the immune system to produce effective antibodies against the virus.
Sources:
Johns Hopkins University Center for Systems Science and Engineering.
https://www.covid19treatmentguidelines.nih.gov/therapeutic-management/
https://www.pharmacytimes.com/news/forecast-predicts-329-drug-spend-increase-in-health-systems.
https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html.






Leave A Comment